Steps to treat patient with diabetic muscle infarction.
HPI
A 48-year-old obese female with past medical history of poorly controlled diabetes, hypertension, hyperlipidemia and hypothyroidism presents to the emergency department (ED) with a four-day history of constant and worsening left upper thigh pain.
She describes the pain as sharp, throbbing, and localized to left medial thigh. Her pain has gradually worsened over past 4 days, with severe tenderness. She has been unable to ambulate for the last two hours. She denies any recent trauma and has not taken any medications for pain. Nothing relieves the pain, which is exacerbated with movement and touch. She denies any associated fever, cough, rash or SOB.
Patient went to PCP earlier today and had bloodwork and U/S doppler of the left leg, which was negative for DVT. Subsequently, she was sent to the ED for further workup. Of note, her A1c in the office today was 9.6, which was down from 13.3 three months ago. Her diabetes is treated with canagliflozin, metformin and PRN insulin glargine.
Pertinent exam findings:
VS: Afebrile, HR 89, BP 142/91, RR 22, 93% on RA
General Appearance: Unable to ambulate, brought back in wheelchair. Significant distress secondary to leg pain.
MSK:
RLE: nontender without any signs of erythema, ecchymosis or rash. No lesions. No peripheral edema and no calf tenderness.
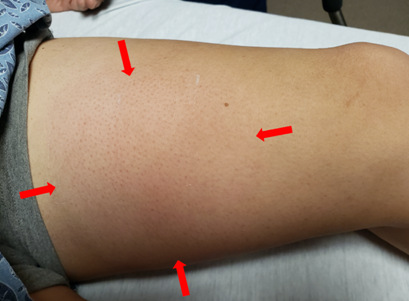
LLE: Approximate 6cmx6cm exquisitely tender area on left medial thigh with overlying edema, erythema and fluctuance; no cyanosis, no rashes, no lesions, no peripheral edema.

Photo of patient’s left medial thigh at initial presentation with 6×6 cm significantly painful area of swelling and erythema (red arrows).
IMAGING:

Figure 2: CT W/ Contrast Sagittal Plane of LLE. There is significant edema within subcutaneous fat space over anteromedial and distal aspect of left thigh (yellow arrows). Hypodensity within subjacent quadricep muscle and vastus medialis obliques muscle (blue arrows). No loculated collection. Perifascial edema around sartorius muscle at same level. No soft tissue air. Bony structures normal. No lymphadenopathy.

Figure 3: CT W/ Contrast Axial Plane of LLE. Refer to figure 2 for same findings and arrow keys.

Figure 4: MRI W/ & W/O CONTRAST T1 Fat Sat Axial view Left Upper Thigh. There is extensive subcutaneous edema over anterior and medial aspect of distal thigh (yellow arrows). Subjacent extensive high signal throughout vastus medialis muscle (blue arrows). Postcontrast images with hypointensity along superficial medial aspect of the vastus medialis muscle, possibly an area of myonecrosis. No soft tissue air. Circumferential perifascial edema surrounding sartorius muscle. Normal bone marrow and posterior compartment musculature is normal.

Figure 5: MRI W/ & W/O CONTRAST T1 Fat Sat Axial View Left Upper Thigh. Refer to Figure 4 for same findings and arrow keys.
CT L LEG THIGH/UPPER LEG W/ CONTRAST
IMPRESSION:
Lower extremity CT shows prominent edema within the distal medial left thigh with hypodensity in the subjacent thigh, most likely diabetic myonecrosis or myositis. Necrotizing soft tissue infection cannot be excluded. Recommended close clinical follow-up.
MRI LLE W/ & W/O CONTRAST T1 fat sat
IMPRESSION:
Findings most in keeping with diabetic myositis with myonecrosis. Deep necrotizing soft tissue infection is an alternative diagnosis, which should be excluded clinically. Recommend close clinical follow up.
DATA INTERPRETATION
CBC: Leukocytosis to 15.9 with left shift (82.8% neutrophils), no anemia or platelet abnormalities
CMP: hyperglycemia to 361, alk phos elevated to 146, total protein elevated to 8.8, otherwise all WNL. CK normal at 100. Kidney function WNL.
ESR: elevated to 25
CRP: elevated to 2.1
Lactic acid: elevated to 3.4
UA: Glucose >1000mg/dL, otherwise WNL
ED Course:
Given 30mL/kg liter IV bolus, Hydromorphone, and ondansetron.
Ordered blood cultures, urine cultures and initiated meropenem and zosyn for suspected infection with allergy to penicillin.
Ordered CT w/ contrast L Leg Thigh/Upper leg.
A general surgeon was consulted initially before CT results due to concern for nec fasc. He saw and evaluated the patient in ED. Although the working diagnosis was diabetic myositis, he stated he would follow along a as a consult.
Hospital Course
Toradol added to and hydromorphone for pain control on the floor, MRI of the lower left extremity with and without contrast ordered. She was continued on her outpatient levothyroxine, simvastatin and lisinopril. Lactic acid improved to 1.2 from 3.4 with fluids.
Her pain, skin changes and L leg weakness improved overnight. MRI resulted confirming suspicion for diabetic myositis with myonecrosis.
She was transitioned to a five-day course of Keflex and outpatient diabetic medications were restarted. Urine and blood cultures pending at time of discharge. The surgeon had no further recommendations. She was advised to take Tylenol as needed for pain. She was prescribed 162mg aspirin daily indefinitely. She was discharged with close follow up with PCP for continued diabetic management with goal A1c of 7.
Discussion
Background
Diabetic muscle infarction (DMI), also known as spontaneous diabetic myonecrosis, describes spontaneous ischemic necrosis of skeletal muscle. DMI was first reported in 1965, but since then only case reports and a systemic review of these cases have been published. This condition is associated with long-standing and poorly controlled DM.[1] The average age of presentation is 40 years and is more common in women and type 1 diabetics.[1-3]
The exact pathophysiology is unknown, although the source of skeletal muscle injury is thought to be secondary to hypoxia-reperfusion injury, atherosclerotic occlusion or vasculitis with thrombosis.[3, 4] Many cases have associated microvascular disease, primarily nephropathy.[1]
DMI presents with atraumatic acute or subacute mild to severe pain with associated swelling to the affected muscle.[1] The affected area may also be indurated and erythematous, and constitutional symptoms are usually absent.[1, 5] DMI most commonly affects a lower limb and unilaterally: quadriceps (~60%), hip adductors (~15%), hamstrings (~10%) and hip flexors (~2%).[3, 4]
Diagnosis
Diagnosis is typically made after first excluding more potentially life and limb threatening issues like necrotizing fasciitis, pyomyositis, abscess, cellulitis and compartment syndrome. Routine bloodwork is typically relatively nonspecific. Total leukocyte count, CK and ESR levels may be normal or elevated.[1] MRI is considered the diagnostic imaging tool of choice[6] while muscle biopsy is not required for diagnosis.[2]
Radiographic features found on CT include diffuse muscle enlargement with decreased attenuation and hyperattenuating subcutaneous fat.[1] Findings on MRI are generally nonspecific with a mass-like area of muscle necrosis along with diffuse fascial and subcutaneous tissue edema.[1, 7] In a T1 weighted image, the affected muscles will appear isointense to hypointense.
In a T2 weighted image, the involved muscles will appear enlarged with diffuse increased signal suggestive of edema as well as subcutaneous edema and subfascial fluid. A post-gadolinium image will consist of diffuse heterogenous enhancement with low-signal, nonenhancing foci that may represent areas of necrosis.[1, 7]
The most accurate diagnostic tool is a tissue biopsy although it is rarely needed. It may be used in some cases of diagnostic uncertainty, but carries the risk of delayed wound healing. [3]
Management in the ED
There is no clear standard of care for managing patients with DMI, [6] which typically responds well to conservative treatment and is self-limiting.[1] One study showed that patients recovered as early as within 5.5 weeks utilizing treatment with antiplatelet agents (i.e. low dose aspirin or clopidogrel) and/or anti-inflammatory drugs (i.e. NSAIDs or steroids).[5]
Supportive treatment, which included rest and analgesics, showed an average recovery time of eight weeks. Surgical excision appeared to have the longest recovery time of 13 weeks.[5] Another study concluded that a combination of bed rest, glycemic control and NSAID therapy yielded the shortest time to symptom resolution and the lowest risk of recurrence.[6] Most patients suffering from DMI likely have comorbidities and/or diabetic complications that are relative contraindications to NSAID treatment.
Prognosis
DMI has a very good short-term prognosis in which patients respond within a few weeks with conservative treatment.[1] However, there is a very high (>50%) recurrence rate reported with as many as one to two episodes per year after the initial event.[1,2]
DMI has a good short-term prognosis with most patients responding to conservative treatment within weeks to months. Unfortunately, approximately 50% of patients with DMI will go on to have an additional episode.
Most patients die within five years of diagnosis, as DMI suggests a substantial vascular compromise. [5] Patients with DMI likely have comorbidities and/or diabetic complications in light of their long-standing and poorly controlled diabetes that has likely contributed to or stemmed from their generalized poor microvascular function.
Clinical Pearls
- Diabetic myositis and myonecrosis should be considered in the differential diagnosis for diabetic patients presenting with extremity pain and swelling
- Early recognition of diabetic myonecrosis is key
- MRI serves as the best tool for diagnosis
- Treatment involves supportive care, glycemic control, and NSAIDs or antiplatelet therapy
- DMI has a good short-term prognosis although a very poor long-term diagnosis
References
- Choudhury, B., et al., Diabetic myonecrosis: An underreported complication of diabetes mellitus. Indian Journal of Endocrinology and Metabolism, 2011. 15(5): p. 58-61.
- Sran, S., et al., Diabetic Myonecrosis: Uncommon Complications in Common Diseases. Case Reports in Endocrinology, 2014. 2014: p. 175029.
- Bhasin, R. and I. Ghobrial, Diabetic myonecrosis: a diagnostic challenge in patients with long-standing diabetes. Journal of Community Hospital Internal Medicine Perspectives, 2013. 3(1): p. 20494.
- Hoyt, J.R. and C.M. Wittich, Diabetic Myonecrosis. The Journal of Clinical Endocrinology & Metabolism, 2008. 93(10): p. 3690-3690.
- Kapur, S. and R.J. McKendry, Treatment and outcomes of diabetic muscle infarction. J Clin Rheumatol, 2005. 11(1): p. 8-12.
- Horton, W.B., et al., Diabetic muscle infarction: a systematic review. BMJ open diabetes research & care, 2015. 3(1): p. e000082-e000082.
- Kattapuram, T.M., et al., Idiopathic and diabetic skeletal muscle necrosis: evaluation by magnetic resonance imaging. Skeletal Radiology, 2005. 34(4): p. 203-209.