Chief complaint of “abscess.” Quick and dirty. Otherwise-healthy patient with a 4 cm abscess on the right thigh with no surrounding cellulitis. No IV drug use history. No significant co-morbidities, and no previous abscesses. Diagnosis: uncomplicated superficial cutaneous abscess (SCA). You know that antibiotics are probably not necessary after I & D. However, the patient complains of pain when you begin to pack it with iodoform gauze. He asks if packing is really necessary. Is it?
The Setup:
Chief complaint of “abscess.” Quick and dirty. Otherwise-healthy patient with a 4 cm abscess on the right thigh with no surrounding cellulitis. No IV drug use history. No significant co-morbidities, and no previous abscesses. Diagnosis: uncomplicated superficial cutaneous abscess (SCA). You know that antibiotics are probably not necessary after I & D. However, the patient complains of pain when you begin to pack it with iodoform gauze. He asks if packing is really necessary. Is it?
The Choices:
1. Yes. More pain med. Inform the patient that packing really does make a difference in preventing abscess reformation.
2. Yes. No pain med. Pack faster and distract the patient with your sense of humor.
3. I’m not sure. Remove the packing and send the patient home with close follow-up.
4. Wing it. Replace the iodoform gauze with sterile packing strips and continue packing.
The Bottom Line:
This is only a pilot study, but it suggests that failure to pack SCA’s will not increase abscess recurrence and will reduce patient suffering. Larger studies are underway to confirm these findings, but for now, post-I&D packing of SCA’s is of questionable benefit to promote rapid wound healing or prevent abscess recurrence.
The Background:
SCA’s are increasingly common in ED’s everywhere. In 1996, U.S. ED’s cared for 1.2 million abscesses compared with 3.3 million visits in 2005. Abscess numbers are increasing far faster than the total number of ED visits, which increased from 90- to 115-million during the same interval.
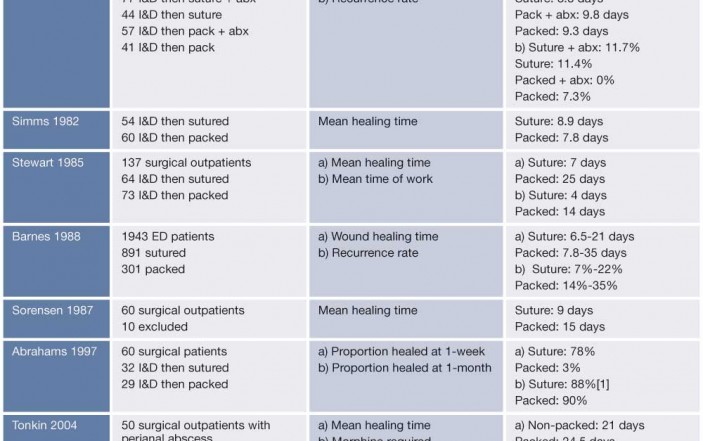
Post-I&D wound-packing may be a long-standing myth. Even well-recognized EM procedural textbooks offer no references to support the “packing doctrine”. In fact, at least seven prior controlled studies suggest that packing prolongs wound healing times without decreasing abscess recurrence rates (Table 1). Caveats: Each of these trials is under-powered with incomplete blinding or randomization schemes and most compare suturing the abscess cavity closed compared with packing. The largest proportions of subjects in these prior trials were ED patients with superficial cutaneous abscesses, but others involved perianal abscess management by surgical consultants in non-ED settings. Dating back to 1951, a substantial number of observational trials have similarly suggested that mixed abscesses heal just as well with primary closure (sutured obliteration of the abscess cavity) as they do with traditional packing.
Macfie 1977, Simms 1982, Stewart 1985, Barnes 1988, Sorensen 1987, Abrahams 1997, Tonkin 2004
The insufficient evidence to support packing aside, there are multiple reasons to consider not packing wounds. First, packing abscesses after routine I&D is painful and EPs often fail to provide sufficient pain control already. One potential solution to alleviate I&D related suffering is procedural sedation with ketamine or propofol in conjunction with systematic analgesia. Second, maternal wound packing with iodoform gauze has been linked to transient infant hypothyroidism so there are risks involved with this traditional post-I&D recommendation.
The objective of the current study was to determine whether the routine and often painful packing of simple cutaneous abscesses is beneficial following I&D.
Results:
48 patients with SCA were randomized with 23 receiving packing (PK) and 25 with no packing (NP). The patients were largely African Americans with no differences in age or sex between the groups. Abscesses were located on the buttock (25%), forearm (19%), or abdominal wall (15%) with the remainder on the thigh, leg, chest or back. Swabs were performed in 83% of patients with 28% positive for bacteria and 73% positive for methicillin-resistant Staphlyococcus aureus (MRSA).
 Two-thirds of the patients were evaluated at 48-hours. Twenty-one patients were from the PK group and 13 were from the NP group. The primary end point was the need for intervention (extension of the incision, further probing, irrigation, packing of the wound, change of initial antibiotics, need for surgical evaluation, admission to hospital or need for another follow-up visit to the ED) at 48-hrs by a blinded attending physician. A total of 9 subjects needed an intervention at the follow-up visit (see Table 2).
Two-thirds of the patients were evaluated at 48-hours. Twenty-one patients were from the PK group and 13 were from the NP group. The primary end point was the need for intervention (extension of the incision, further probing, irrigation, packing of the wound, change of initial antibiotics, need for surgical evaluation, admission to hospital or need for another follow-up visit to the ED) at 48-hrs by a blinded attending physician. A total of 9 subjects needed an intervention at the follow-up visit (see Table 2).
Of the 14 no-shows there were 11 from the NP group and 3 from the PK group. Although the authors do not report a p-value for this differential follow-up rate, one can use online statistical calculators to do so independently and the difference is significant (p = 0.03). However, the investigators were able to reach 10 of the 11 from the NP group by telephone. These patients reported no pain and did not think it was necessary to return to the ED for re-evaluation. Only 1 of the 3 from the PK group lost to follow up was contacted by phone. They reported moderate pain, but did not return due to lack of transportation. Seventy-five percent (36/48) of the patients were contacted by telephone between day 10 and 15 (17/24 PK and 19/24 NP) and none reported any complications or additional interventions.
Pain was assessed in this study using the standardized visual analog scale (VAS). There was no difference in VAS pre-procedure between the two groups. However, immediately post-procedure the PK group reported pain scores which were significantly higher (difference of means = 23.8mm, 95% CI=5 to 42 mm, p=0.014). The PK group also reported higher pain scores at the 48 hour follow-up (difference of means = 16.4mm, 95% CI = 1.6 to 31.2mm, p=0.03). There was no difference between the groups in use of ibuprofen. However, patients in the PK group used more oxycodone/acetaminophen pills (mean 3.1 compared to 0.91, p=0.03).
Caveats:
This study excluded abscesses larger than 5cm, pregnancy, co-morbid medical conditions including diabetes, HIV o
r any malignancy, chronic steroid use, immunosuppressive states including but not limited to sickle cell disease and sarcoidosis, abscess located on the face, neck, scalp, hands, feet perianal, rectal or genital areas, hidradenitis or pilonidal abscesses and need for procedural sedation or supplemental treatment (IV antibiotics or surgical consult which may limit its application to busy EDs.
The authors recognize that the study was too small to have their results generalized. At least two clinical trials are currently underway to address this issue (at NYU and StonyBrook). However, with abscess management an increasingly prevalent problem for busy EPs, avoiding post-I&D packing could simultaneously decrease patient procedural suffering while decreasing ED follow-up visits for re-packing.
Case Outcome:
You explain to the patient that the incomplete data doesn’t support packing a SCA after I & D, but you’ll let him decide. Duh? He decides to forego wound packing. You discharge him with instructions to return in 2-days for a wound re-evaluation wondering whether the routine follow-up visit might someday be proven another clinical care myth unnecessarily clogging our EDs.
Dr. Milne is an adjunct professor of emergency medicine at the University of Western Ontario, and is a BEEM Faculty Member, McMaster University




11 Comments
If packing and antibiotics of questionable value in an uncomplicated SCA, what about routine wound culture too?
To bad there was no mention about MRSA SCA. Anecdotally 2/3rd of patients seen by me in clinic and ED settings are +. These Abscess are notoriously purulent. Iodoform wick provides outlet until drainage stops. In clinic setting am able to follow in three days and will only repack if drainage is present. I have incised and drained literally 100’s of these abscess in clinic and virtually all have done well with this routine and Bactrim or Doxy and sometimes Augmentin if grossly cellulitic and/or febrile initially
Although the blunt pressure of the placement of the packing itself does cause some discomfort, I find it is the iodoform that causes the most discomfort, while plain packing does not. I also inform the patient (assuming some intelligence exists) that if the wound looks good, that they can remove the packing themselves and only return if the area looks/feels worse.
If it is felt that a wide incision is not enough to provide adequate drainage, then just insert a small gauze wick to hold the incision open. The term “packing” should be avoided since it implies that the abscess cavity needs to be stuffed with gauze, when such packing of an abscess may actually impede drainage. A wide and adequate incision followed by frequent soaks is probably all that is ever required for treating the vast majority of simple abscesses.
Why does no one suggest topical anesthesia, such as lidocaine applied 5 minutes before packing? I’m a veterinarian and have had to pack my breast this week, and was stunned when no one suggested topical pain killer. I had bupivicaine, so used that before I tried it the next time. It made a huge difference. Seems like a no brainier to me…
I just had an abscess packed after I&D with no pain because they did a single quick injection of lidocaine. As a patient this was appreciated.
Great review and discussion.
In wound care it is a standard of care to fill dead space to prevent premature closure of the wound. However, is the packing material the root of the problem causing pain, decreased healing times, and complications?
Please refer to Advances in Skin and Wound Management January 2013 for a clinical study comparing the materials for abscess mgmt post I&D.
Keep the baby (Standard of care), throw out the bath water (100+ year old materials like gauze and iodoform gauze).
My recent abscess was MRSA positive and my EP chose not to pack, and offered my abx if needed. They weren’t. Healing was quick, and no recurrent infxn. Happy he was interested in current research.
8 cm abcess in left breast drained by surgeon on Jan. 28th, 2015 at Maui Memorial hospital. Still packing 1/4 gauze in wound as of today, March 10th. It’s getting painful as wound is healing. My surgeon is now telling me I could have a pocket below and may have to have surgery again. There is not redness around the wound opening. Breast is soft and not sore except at the a bit below the wound site. Had lumpectomy for breast cancer Dec. 1, 2014. No complications then, wound up in hospital after airplane flight to Maui. Doc there said I had a staph infection and put me on a antibiotic drip for 4 days. Then sent me home with 10 days of oral Cipro. Wound is healing well. Why are they worried about a pocket and threatening me with more surgery.
I hope you can help me. I am 71 yrs old.
Sorry, I am only a patient whose husband is packing her wound. I may not be eligible for a response to my question just sent on May 10, 2015 at 9:28 p.m.
I had a abcess boil on upper buttocks, I went to Er they had to lance it. Well when Doc. tried to numb it he said it might not work because the boil to hard and big. That was the worse pain I’ve ever felt in my life, I screamed, cried, cursed my left leg was shaking then lwent numb for a few seconds. Doc. never stopped then he packed it with so much gauze it burned . The pain from boil is so much better but the pain I have now I feel like is from gauze, when it come out slowly it hurts like a shooting pain. Its not swollen little red and has no smell. Its been three days can I take the rest of gauze out? Thanks