A real-world guide to pyogenic flexor tenosynovitis.
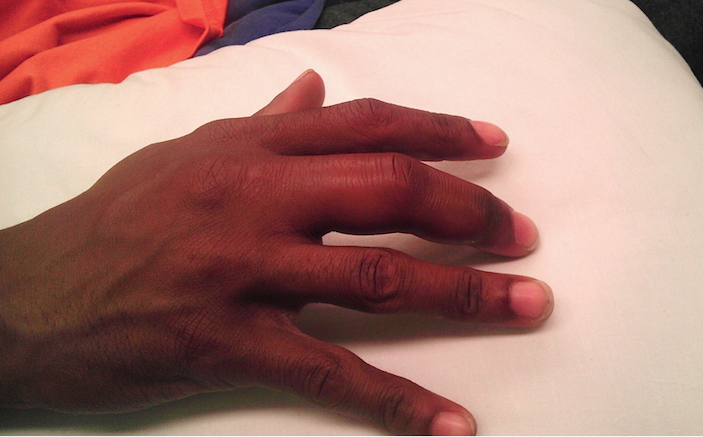
A 24-year-old, healthy, left-hand dominant male presented to the emergency department (ED) for pain and swelling in his right third finger. He stated that he had been picking at a wart on the distal end of the finger and that approximately two days ago, he began noticing progressive pain and swelling, prompting his ED visit. He complained of severe pain localized to this finger and difficulty with range of motion, but denied numbness, tingling, fevers, or trauma to the digit. His vitals were temperature 98.1F, HR 80, BP 116/65, and resp 16. Physical examination revealed a swollen right third finger with mild erythema, painful passive extension and flexion, and a small visible verruca on the volar aspect (Image 1 above and image 2 below). Palpation caused significant pain throughout, more notably on the volar aspect of the finger. Other than his finger, his physical examination was unremarkable.
 Image 2 – Finger held in slight flexion. Small verruca at interphalangeal crease is appreciable
Image 2 – Finger held in slight flexion. Small verruca at interphalangeal crease is appreciable
Plain radiographs did not show a foreign body, fracture, or dislocation. The absence of focal swelling made felon or abscess less likely. Laboratory evaluation was unremarkable with a white blood cell count of 10,200 and normal differential. Based on the observation of a wound overlying the flexor tendon (and thus a site of possible bacterial inoculation) in the presence of all four Kanavel signs, a clinical diagnosis of pyogenic flexor tenosynovitis was made. Intravenous antibiotics were started and the orthopedics service was consulted for operative management. The patient was taken from the ED directly to the operating room for irrigation where pyogenic flexor tenosynovitis was confirmed by copious expression of purulent fluid from the tendon sheath. He underwent repeat irrigation the next day and was discharged on a regimen of oral antibiotics.
Background
Pyogenic flexor tenosynovitis (PFT), also known as infectious flexor tenosynovitis, is an acute infection of any tendon sheath. PFT is an aggressive process that can lead to debilitating comorbidity for patients: decreased range of motion, loss of movement, or possibly amputation [1-3]. The infection spreads through the tendon sheath, which consists of a visceral and parietal layer. In their physiologic state, these sheaths are closed at the ends, creating a sealed compartment that encases the tendon itself. The flexor tendon sheaths of the second, third and fourth digit tend to be confined to those fingers alone, however the thumb and little finger sheaths commonly join to the radial and ulnar bursae, respectively [3]. When bacteria are introduced into this closed space by traumatic injury such as animal bites, puncture wounds (most common), or hematogenous spread, purulent and inflammatory materials fill the layers of this space [4]. Due to the poor vascularity of tendon sheaths, bacteria are allowed to rapidly proliferate once within them, causing increased pressure that can eventually lead to necrosis and possible rupture of the tendon sheath [3]. The most common offending bacteria are Staphylococcus and Streptococcus species, however depending on the source of inoculation (e.g. dog/cat bites, human bites, other contamination, etc.), other bacteria such as as Pasteurella, Eikenella, or Fusobacterium species may be involved [1,3-5].
PFT can be an elusive diagnosis, thanks to several infectious and inflammatory conditions of the digits that can mimic the signs and symptoms. These include, but are not limited to, cellulitis, abscesses, gouty arthritis, psoriatic arthritis, felons, septic arthritis, herpetic whitlow, and trauma [1,3]. It is also difficult to differentiate superficial versus deep space infections in the hand as its anatomy is complex and the deep spaces miniscule. For these reasons, a high degree of clinical suspicion is necessary to get patients with PFT to the definitive treatment of antibiotics and incision and drainage (I&D).
Diagnosis
Although the existence of PFT has been described in medical literature since at least 1912 when American surgeon Allen Kanavel described his three cardinal signs (now four), its diagnosis continues to elude practitioners [6]. A high degree of suspicion helps to lead to the clinical diagnosis. PFT diagnosis is largely based off Kanavel’s cardinal signs: 1) tenderness along the tendon sheath, 2) fusiform swelling of the digit, 3) finger held at slight flexion at rest, and 4) pain on passive extension. While still a commonly used tool in clinical diagnosis, the sensitivity, specificity, and inter-observer reliability of this tetrad have not been established in the literature [1]. Presence of Kanavel’s signs varies case by case and there is no agreement as to which signs are the most important in making the diagnosis of PFT. In analysis of 75 patients with PFT, Pang et al. found that 97% had fusiform swelling, 72% pain on passive extension, 69% held finger at slight flexion at rest, and 64% had tenderness along the tendon sheath [7]. Conversely, Dailiana et al. found that 100% of their patients had tenderness along the tendon sheath and pain with passive extension in their study of 41 patients with PFT. Additionally, they found that only 54% of patients with PFT had all four Kanavel signs [8]. With disagreement between numbers, finding a reliable diagnostic pattern is difficult at best. Magnetic resonance imaging (MRI) may be helpful in the diagnosis of some soft tissue infections, however this modality’s utility may be impeded by cost, availability, and time. Laboratory assessment is also not very helpful in establishing a definitive diagnosis. Bishop et al. showed that while elevations in the WBC, ESR, and CRP may support the diagnosis when already suspected, the negative predictive value of these markers are 4%, 3%, and 13%, respectively, and thus cannot be used to rule out infection [9]. While we struggle to correlate non-specific labs with the utility of century-old diagnostic criteria for diagnosing PFT, the time to use a common everyday tool may be at hand (pun intended).
Bedside Ultrasound in the Diagnosis of PTS
In many EDs, and especially academic EDs, we use ultrasound daily or even hourly to aid in the rapid diagnosis of many different conditions. Congestive heart failure, pneumonia, abdominal aortic aneurysm, deep vein thrombosis, retinal detachment, pulmonary embolism, cholecystitis, and intra-abdominal hemorrhage are several of these conditions. Additionally, some of the best applications for ultrasound lie in the diagnosis of soft tissue conditions such as foreign body, abscess, and necrotizing skin infections. It’s time to add PFT to this list.
Ultrasonography has been shown to easily identify soft tissue infection even in novice sonographers. Berger et al. demonstrated that with only minimal ultrasound training, practitioners were able to identify soft tissue purulent collections that would be amenable for incision and drainage more successfully than by clinical judgment alone [10]. While this study addressed purulent fluid pockets in tissues (abscesses), the same principle can be applied to the tendon sheath.
When attempting to identify superficial structures such as the tendon sheath, the ideal probe to utilize is the linear probe due to its high frequency, which allows for higher resolution images [11-13]. There can be some constraints with use of a linear probe – it can be difficult to obtain direct skin contact with the involved digit, as PFT may cause the digit to assume a flexed position, not to mention direct pressure of the probe along the skin increases pain. In order to resolve both of these issues, one can use a large basin filled with lukewarm water to submerge the entire hand, as the water medium allows better visualization of the involved digit without requiring direct placement of the probe onto skin [12-13].
Tendons appear as hyperechoic, or bright white, structures on ultrasound. Longitudinally, they are striated and anisocoric, while nerves often have a typical “honeycomb” appearance (Images 3-5). Knowing the local anatomy, location of major nerves, and sonographic appearance of nerves is important, but even with this knowledge it can sometimes be challenging to differentiate between a nerve and tendon. The best bedside trick to help you confirm the structure is to drag the linear probe along the tendon, tracking it in cross section proximally up the arm. Tendons will disappear and turn into muscle body, while the nerves remain. Another trick is to passively move the fingers during the ultrasound. Tendons will move in the image while nerves remain stationary. Luckily, flexor tendons in the fingers lie directly volar to the phalanges and are easy to identify. When the tendon sheath has been inoculated with bacteria, the hyperechoic tendon will be surrounded by a hypoechoic or anechoic material representing purulent fluid that increases the suspicion for PFT [14] (Image 6).
 Image 3 – Normal longitudinal view of the tendon (arrow) showing a hyperechoic, striated appearance.
Image 3 – Normal longitudinal view of the tendon (arrow) showing a hyperechoic, striated appearance.
 Image 4 – Comparison between transverse view of tendon and nerve (compare to image 5). Classic “honeycomb” appearance of the median nerve (arrow)
Image 4 – Comparison between transverse view of tendon and nerve (compare to image 5). Classic “honeycomb” appearance of the median nerve (arrow)
 Image 5 – Comparison between transverse view of tendon and nerve (compare to image 4). This image depicts a more hyperechoic tendon (arrow).
Image 5 – Comparison between transverse view of tendon and nerve (compare to image 4). This image depicts a more hyperechoic tendon (arrow).
 Image 6 – Longitudinal view of tendon with PFT, exhibiting the hypoechoic appearance of fluid (star) surrounding the tendon (arrow).
Image 6 – Longitudinal view of tendon with PFT, exhibiting the hypoechoic appearance of fluid (star) surrounding the tendon (arrow).
A small study conducted by Schecter et al. demonstrated that ultrasound depiction of a fluid collection associated with Kanavel’s signs was supportive in diagnosing PFT. Of the 18 patients who presented with a swollen tender finger that were evaluated by bedside ultrasound, 11 (61%) had evidence of fluid collection and were proven to have PFT during surgical irrigation. One patient of the 18 that was highly suspected of having PFT did not demonstrate a fluid collection on bedside ultrasound, and showed no obvious purulent drainage upon surgical exploration [15]. Although the sensitivity or specificity of this noninvasive, radiationless, and readily available method has yet to be determined, these studies show the ease and rapidity with which ultrasonography can be used to aid in quick diagnostic evaluation for a potentially debilitating condition.
Treatment
PFT is an orthopedic emergency treated by intravenous antibiotics and operative I&D, either by open method or catheter irrigation technique, the latter which may be associated with improved digit mobility [2]. Once the diagnosis is seriously entertained, antibiotics covering Staphylococcal and Streptococcal species should be started and orthopedics consulted for I&D. A reasonable combination of antibiotic coverage includes ciprofloxacin or a third generation cephalosporin such as ceftriaxone, in addition to vancomycin. Another feasible regimen is ampicillin/sulbactam, cefoxitin, or piperacillin/tazobactam in combination with vancomycin [5]. Vancomycin is especially important to include if methicillin-resistant Staphylococcus aureus (MRSA) is suspected based on clinical factors, such as a history of intravenous drug abuse, known prior MRSA infection, immunocompromise, or community bacterial profiles [4]. If there is concern for animal bite contamination, treatment should be directed towards Pasteurella species, with penicillin G, ampicillin/sulbactam, fluoroquinolones such as levofloxacin, piperacillin/tazobactam, or higher generation cephalosporins including ceftriaxone or cefixime plus metronidazole [16-17]. With human bites, coverage should be directed towards Eikenella or Fusobacterium species with ampicillin/sulbactam or piperacillin/tazobactam [18]. If orthopedics is not available or the diagnosis is in question, the patient should be transferred to a facility with the appropriate resources, as misdiagnosis can increase the likelihood of complications.
Conclusion
As technology plows forward, there still does not exist a proven method for diagnosing PFT. Ultrasound has become our gold-standard bedside assistant in providing us clues for rapid diagnoses and this holds true for PFT as well. Dr. Kanavel would probably be happy to know that the signs he described 104 years ago are still kicking, but one can imagine the dismay at knowing we are still working on finding a definitive method for diagnosing PFT. While only 54% of patients with PFT may have all four signs, the finding of any of these signs should add PFT to our hypothetical imaginary lists of must-rule-outs. PFT is a tricky diagnosis with potentially severe outcomes. Although bedside ultrasound may be a welcome addition to our diagnostic arsenal, it is still important to remember the basics!
Special thanks to Dr. Gavin Budhram for providing high quality ultrasound images.
REFERENCES
- Kennedy, Colin D., Jerry I. Huang, and Douglas P. Hanel. “In Brief: Kanavel’s Signs and Pyogenic Flexor Tenosynovitis.” Clinical Orthopaedics and Related Research 474.1 (2016): 280–284. PMC. Web. 21 Sept. 2016.
- Giladi, A. M., S. Malay, and K. C. Chung. “A Systematic Review of the Management of Acute Pyogenic Flexor Tenosynovitis.” Journal of Hand Surgery (European Volume) 40.7 (2015): 720-28. Web.
- Patel, Dakshesh B., Neelmini B. Emmanuel, Milan V. Stevanovic, George R. Matcuk, Christopher J. Gottsegen, Deborah M. Forrester, and Eric A. White. “Hand Infections: Anatomy, Types and Spread of Infection, Imaging Findings, and Treatment Options.” RadioGraphics 34.7 (2014): 1968-986. Web.
- Sexton, Daniel J., MD. “Infectious Tenosynovitis.” Infectious Tenosynovitis. N.p., n.d. Web. 20 Sept. 2016.
- Tintinalli, Judith E., and J. Stephan. Stapczynski. “Nontraumatic Disorders of the Hand.” Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 7th ed. New York: McGraw-Hill, (2011): 1920-1926. Print.
- Kanavel AB. The symptoms, signs, and diagnosis of tenosynovitis and fascial-space abscesses. In Infections of the Hand. 1st ed. Philadelphia, PA: Lea & Febiger; 1912:201–226. Print.
- Pang, Hee-Nee. “Factors Affecting the Prognosis of Pyogenic Flexor Tenosynovitis.” The Journal of Bone and Joint Surgery (American) J Bone Joint Surg Am 89.8 (2007): 1742-1748. Web.
- Dailiana, Z. H., N. Rigopoulos, S. Varitimidis, M. Hantes, K. Bargiotas, and K. N. Malizos. “Purulent Flexor Tenosynovitis: Factors Influencing the Functional Outcome.” Journal of Hand Surgery (European Volume) 33.3 (2008): 280-85. Web.
- Bishop, Gavin B., Trevor Born, Sanjeev Kakar, and Andrew Jawa. “The Diagnostic Accuracy of Inflammatory Blood Markers for Purulent Flexor Tenosynovitis.” The Journal of Hand Surgery 38.11 (2013): 2208-211. Web.
- Berger, Tony, Francisco Garrido, Jeffrey Green, Penelope Chun Lema, and Jay Gupta. “Bedside Ultrasound Performed by Novices for the Detection of Abscess in ED Patients with Soft Tissue Infections.” The American Journal of Emergency Medicine 30.8 (2012): 1569-573. Web.
- Szabo, Thomas L. “Transducer Arrays for Medical Ultrasound Imaging.” Ultrasound in Medicine 32.4 (2013): 573-82. Web.
- Krishnamurthy R, Hyun Yoo J, Thapa M, Callahan M. Water-bath method for sonographic evaluation of superficial structures of the extremities in children. Pediatr Radiol. 2013 Mar;43 Suppl 1:S41-7. doi: 10.1007/s00247-012-2592-y. Epub 2013 Mar 12.
- Blaivas M, Lyon M, Brannam L, Duggal S, Sierzenski P. Water bath evaluation technique for emergency ultrasound of painful superficial structures. Am J Emerg Med 2004 Nov;22(7):589-93.
- Ihnatsenka, Barys, and André Pierre Boezaart. “Ultrasound: Basic Understanding and Learning the Language.” International Journal of Shoulder Surgery 4.3 (2010): 55–62. PMC. Web. 21 Sept. 2016.
- Schecter, William P., Robert E. Markison, R. Brooke Jeffrey, Ronald M. Barton, and Faye Laing. “Use of Sonography in the Early Detection of Suppurative Flexor Tensosynovitis.” The Journal of Hand Surgery 14.2 (1989): 307-10. Web.
- Weber, David J., MD, MPH, William A. Rutala, PhD, MPH, and Sheldon L. Kaplan, MD. “Pasteurella Infections.” Pasteurella Infections. N.p., n.d. Web. 27 Sept. 2016
- Baddour, Larry M., MD, FIDSA. “Soft Tissue Infections Due to Dog and Cat Bites.” Soft Tissue Infections Due to Dog and Cat Bites. N.p., n.d. Web. 20 Sept. 2016.
- Baddour, Larry M., MD, FIDSA. “Soft Tissue Infections Due to Human Bites.” Soft Tissue Infections Due to Human Bites. N.p., n.d. Web. 20 Sept. 2016.