Unique exam findings in handling progressive pain.
A 68-year-old male with a past medical history of poorly controlled type 2 diabetes mellitus (A1c 9.1), coronary artery disease, chronic obstructive pulmonary disease, hypertension and hyperlipidemia, presented to the ED with the chief complaint of left shoulder pain for the last 14 days.
The patient had a history of prior left rotator cuff repair in 2019 complicated by infectious left shoulder bursitis (reconstructed supraspinatus tendon) in August 2020 with joint aspiration positive for S. Aureus requiring a four-week course of intravenous Daptomycin.
He was seen in the ED 11 days prior with left shoulder pain after he picked up a garbage bag and heard a “crack.” X-rays of the shoulder at that time were unremarkable and showed stable suture anchors from his prior rotator cuff repair. He was discharged home and one week later presented to an orthopedic clinic with worsening left shoulder pain. He was started on Bactrim due to his history of infective bursitis and prior cultures showing susceptibility.
Three days later, the patient presented again to the ED with persistently worsening pain – now rated a 7/10 in severity and a newly noted mass with surrounding skin changes to the anterior left shoulder. The patient denied any systemic symptoms including fevers, chills, nausea or vomiting. He also denied any drainage from the wound.
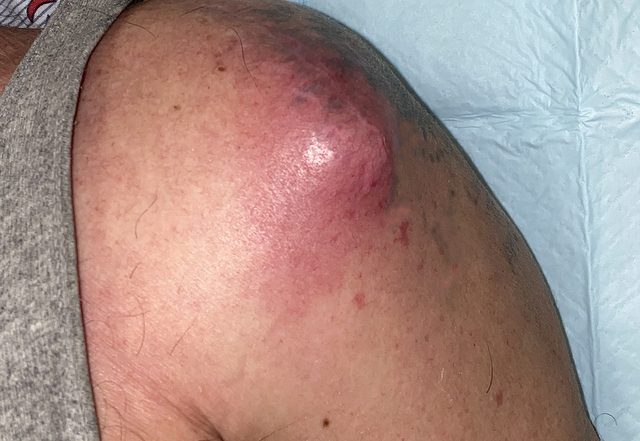
On initial exam, his vitals were: BP 126/62 mmHg, HR 98 bpm, RR 18, SpO2 98% on room air, and temperature of 36.9°C. The patient was well appearing and in no acute distress. He had swelling and mild tenderness to the left anterior shoulder: there was a 4 x 4.5 cm area of erythema and fluctuance to the anterior shoulder (figure 1) that was not present on prior exams.

The area of fluctuance was noted to be over the previous anterior surgical scar. He was otherwise neurovascularly intact in the left upper extremity with normal range of motion of the left shoulder.
Initial labs revealed no leukocytosis, but an ESR elevated to mm/hr and CRP of mg/L. Basic metabolic panel was unremarkable. Blood cultures were obtained and later were negative. Bedside US was performed in the emergency department (figure 2a-c) and the orthopedic team was consulted.



Bedside US showed a fluid collection in the area of the erythematous nodule penetrating the muscle and tracking down at least to the joint capsule, possibly intra-articular.
An MRI was ordered, and the patient was admitted to the orthopedic service. MRI results demonstrated presumed glenohumeral joint septic arthritis with dissection of infected fluid into bursal spaces of left shoulder, along the proximal humerus, and through the deltoid muscle forming an abscess in the lateral subcutaneous tissue (figure 3a-b).


Antibiotics were held at this time as the plan was incision and drainage in the operating room. The patient went to the OR the that night and the old surgical wound and new abscess was irrigated and debrided. The anchor and loose suture material were removed and a penrose drain was placed.
Cultures of the purulent material grew out S. Aureus. The patient was started on Ancef. A PICC line was placed, and the patient was discharged home on a six-week course of IV Ancef per infectious disease recommendations. Medicine was also consulted to improve glycemic control. On follow-up orthopedic visit, the patients’ symptoms were much improved following outpatient antibiotics.
Discussion:
Septic arthritis is defined as an infection in the joint fluid and joint tissues. [3,11] The diagnosis of septic arthritis can be challenging in the Emergency Department as there is no specific clinical pattern, although certain general features are often observed such as joint pain (85%), joint swelling (78%), and fever (57%).[3]
Point of care ultrasound can help clinicians characterize joint swelling to facilitate the diagnosis of septic arthritis. [10] Even after joint aspiration there are different thresholds for WBC count and PMNs based on certain patient characteristics (such as history of immunosuppression and known prosthetic joint). The gold standard for diagnosis is synovial fluid culture/gram stain. Inflammatory markers may be utilized; often an ESR threshold of > mm/hr is found to be fairly sensitive for septic arthritis. [4]
Classic teaching is that in a healthy non-prosthetic joint bacterial septic arthritis is consistent with WBC> 50,000 or >90% PMNs.[5] This is highly variable and patient factors should be considered. When considering a prosthetic joint a lower threshold is considered; >10,000 WBC or 90% PMNs.[5]
If joint aspiration is consistent with a septic joint, an orthopedic specialist should be consulted for further management. Treatment involves intravenous antibiotics and/or joint irrigation in the operating room. Septic arthritis is more common in prosthetic joints versus native joints. Incidence of septic arthritis in native joints is noted to be 6-10 cases per 100,000 individuals per year.[6]
The incidence for septic arthritis in prosthetic joints is noted to be much higher at 1,000 to 3,000 cases per 100,000 individuals per year.[9] The knee is the most commonly affected joint in all cases of septic arthritis. [7,8] Our patient presented with septic arthritis in the setting of prior rotator cuff surgery. Overall, septic arthritis is reported after less than 1% of arthroscopy procedures.[1]
Specifically, “after shoulder arthroscopy, infections occur with a frequency of about 0.3%.” [2] Although rare, our patient had multiple co-morbidities, which made him susceptible to infection. Also, at the time of prior rotator cuff repair, some suture anchors were removed, which would allow a communication for superficial/subacromial abscess with glenohumeral joint.
Conclusion:
For any joint pain presenting to the ED, septic arthritis must be in the differential diagnosis. Using history, physical exam and other diagnostic modalities the emergency physician can stratify a patient’s risk of septic arthritis. Ultrasound is a great non-invasive diagnostic modality used in multiple clinical scenarios and has been well described as a modality to diagnose joint effusions. The above-mentioned case is an example of how ultrasound can be used to help assist the orthopedic and emergency medicine team to direct focus on the possibility of septic arthritis, especially in a case where there were few initial clinical signs/symptoms to suggest septic arthritis.
Ultrasound was used specifically in this case to see the extent of a fluid collection that was over a previous surgical scar. It helped determine that the fluid collection was more than just a superficial abscess/collection (figure 2a-c). This case represented a unique case of septic arthritis/bursitis in a previously surgically manipulated joint.
Citations
- Bauer, P. Boisrenoult, J.-Y. Jenny, Post-arthroscopy septic arthritis: Current data and practical recommendations, Orthopaedics & Traumatology: Surgery & Research, Volume 101, Issue 8, Supplement, 2015, Pages S347-S350, ISSN 1877-0568
- Yeranosian MG, Arshi A, Terrell RD, et al. Incidence of acute postoperative infections requiring reoperation after arthroscopic shoulder surgery. Am J Sports Med2014; 42(2): 437–441.
- Burton J, Fortuna Septic arthritis . In: Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 8Th ed. New York: Mc Graw-Hill Education; 2016:1931-1931.
- Li SF, Henderson J, Dickman E, Darzynkiewicz R. Laboratory tests in adults with monoarticular arthritis: can they rule out a septic joint. Acad Emerg Med. 2004;11:276–280
- Burton J, Fortuna Septic arthritis . In: Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 8Th ed. New York: Mc Graw-Hill Education; 2016:1927-1928.
- Nade, S . Septic arthritis. Best Pract Res Clin Rheumatol 2003; 17: 183–200
- Peres, LR, Marchitto, RO, Pereira, GS. Arthrotomy versus arthroscopy in the treatment of septic arthritis of the knee in adults: a randomized clinical trial. Knee Surg Sports Traumatol Arthrosc 2016; 24: 3155–3162.
- Roerdink RL, Huijbregts HJTAM, van Lieshout AWT, Dietvorst M, van der Zwaard BC. The difference between native septic arthritis and prosthetic joint infections: A review of literature. Journal of Orthopaedic Surgery. May 2019.
- Zimmerli, W . Prosthetic-joint-associated infections. Best Pract Res Clin Rheumatol 2006; 20: 1045–1063.
- Firnberg, Maytal T. MD; Rabiner, Joni E. MD. Point-of-Care Ultrasound of a Shoulder Effusion in a Child With Septic Arthritis: A Case Report. Pediatric Emergency Care: February 2022 – Volume 38 – Issue 2 – p e1025-e1027
- Gottlieb, Michael MD; Holladay, Dallas DO; Rice, Melissa MD. Current Approach to the Evaluation and Management of Septic Arthritis. Pediatric Emergency Care: July 2019 – Volume 35 – Issue 7 – p 509-513



1 Comment
The Ancef brand name has been discontinued in the U.S. I am assuming that you administered cefazolin, the generic form of the antibiotic. Your article should state this correctly.