BEEM [2010 Flu Update]
Educational Objectives:
After evaluating this article participants will be able to:
1. Discuss the treatment of influenza and formulate a rational approach to care for influenza patients
2. Incorporate strategies for the use of antiviral agents into clinical practice
3. Develop a plan for appropriate post exposure prophylaxis measures
Although most states are finally catching their breath from the insurgence of H1N1 seen in recent months, the second wave is coming soon. Fortunately, this break in the action provides us with the opportunity to reflect and see what we learned. Tamiflu was flying off the shelves, but did it really work? If so, which patients benefited? Will you participate in the next round of “Tami-mania?” I certainly hope not. Our experiences have given us the opportunity to assess the literature and formulate some rational conclusions. In order to guide you in this quest, we’ve broken the lessons learned into five myths that need busting.
Emergency departments around the country are catching their breath from H1N1, but the second wave is scheduled for the Spring. This break in the action provides us with the opportunity to reflect on this pandemic, the treatments and strategies that were recommended and how we put them into practice. Our experiences have given us the opportunity to assess the literature and formulate some rational conclusions. In order to guide you in this quest, we’ve broken the lessons learned into five myths that need busting.
Myth #1 . Antiviral therapy reduces mortality and morbidity in non-hospitalized healthy adults with influenza
Recently, the efficacy and safety of anti-viral agents have been questioned. In December 2009, Shannon Brownlee, author of the best-seller Overtreated, reported that a 2006 Cochrane Review detailing the potential for oseltamivir to reduce post-influenza complications like bronchitis, pneumonia, and sinusitis was called into question. The Cochrane authors decided to re-analyze the data when it became apparent that only 2 of 10 studies upon which they based their 2006 conclusions had been subsequently published and all were sponsored by the company that manufactured oseltamivir. Their attempts to evaluate the original data were reportedly stymied by the drug companies’ investigators who ultimately provided incomplete data sets that demonstrated far less favorable, even marginal benefit to influenza patients. Since the United States has spent over $1.5 billion stockpiling oseltamivir since 2005 in anticipation of a future pandemic, these concerns are obviously pertinent to more than frontline clinicians. However, Brownlee reports that neither the FDA nor the CDC has called for a conclusive randomized controlled trial (oseltamivir vs. standard of care). So, the efficacy of the anti-viral agent remains in question.
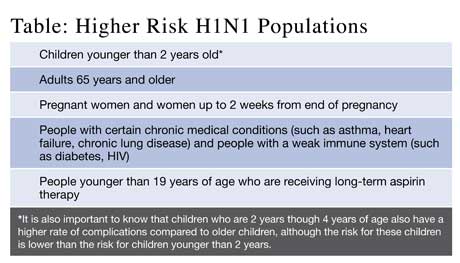
In the 2009 update of the 2006 Cochrane review cited by Brownlee, a meta-analysis of 20 trials (4 prophylaxis, 12 treatment, 4 post-exposure prophylaxis) was performed. Oseltamivir offered post-exposure prophylaxis efficacy of about 58% and 84% in two trials of household contacts with similar performance by inhaled zanamivir. If taken within 48-hours of symptom onset, neuraminidase inhibitors reduced influenza symptom duration by about one-day in outpatients, but no randomized studies support a benefit in critically ill hospitalized patients. No mortality reduction benefit in healthy (see Table) or hospitalized patient populations was reported. Nonetheless, pharmaceutical-sponsored papers suggest a role for these agents in severe influenza. Unfortunately, reports of anti-viral resistant strains are now being reported for both seasonal H1N1 and the pandemic 2009 influenza A H1N1 strains.
Conclusion: Oseltamivir or zanamivir reduce influenza symptom duration if used within 48-hours of symptom onset, but to date no research evidence suggests a mortality benefit for these agents in any population.
Myth #2 . Antiviral prophylaxis or primary treatment in the general population is cost-effective
The United Kingdom Centre for Reviews and Dissemination published an exhaustive economic evaluation of antiviral agents for influenza in 2009. A systematic search extracted empirical data from 29 clinical trials and did suggest that zanamavir and oseltamivir reduce symptom duration by about one-day. One commonly reported measure in cost-effectiveness analyses is the Quality-adjusted life year (QALY) which is a monetary measure of disease burden that measures the impact of a medical intervention on the quality and quantity of life. Assuming that strategies costing less than about $40,000 per QALY are cost-effective (a common assumption in such analyses), and that antiviral treatment does not save lives or prevent complications, the analysis finds that treatment was cost-effective for healthy children and adults only when the prevalence of influenza was greater than 20% among patients with influenza-like illness (ILI).
The societal cost is of course even higher if one must go to the doctor to get the diagnosis and prescription. However, treating patients with risk factors (Table) was considered cost-effective in almost all scenarios. Furthermore, assuming that antiviral use reduces antibiotic use (several studies suggest that they do, especially in pediatric practice) also increased the cost-effectiveness of all antiviral strategies although whether the antibiotics were indicated in the first place could be questioned. It is worth noting that this elaborate analysis with its hundreds of assumptions and sensitivity analyses was created to justify public spending. Its conclusions are easily trumped by an individual patient’s willingness to pay for antivirals, usually due to an exaggerated estimate of personal risk. In addition, medication costs have not been an issue since the federal government released over 500,000 doses of oseltamivir from the Strategic National Stockpile to states in Oct 2009 for free distribution to treat influenza cases. Since the medications are a national asset, pharmacies cannot charge for the pills although they may charge a dispensing fee. You can contact your local health department to learn how to access these free medications within your jurisdiction.
A cost-effectiveness analysis for post-exposure prophylaxis (PEP) with anti-viral agents has also been evaluated. If one assumes that no anti-viral therapy will be initiated once symptom developed, a Canadian analysis demonstrated that PEP with oseltamivir to prevent one case of influenza cost $764, $479, or $137 if the rate of transmission were 8%, 12% or 30%, respectively. They concluded the PEP for family contacts would be a cost-effective strategy from a societal perspective if influenza-like illness cross contamination rates exceeded 8% and the only treatment was symptom control. A British analysis of PEP with antivirals concluded that the available data were insufficient to conclude that they were worthwhile.
Conclusion: The use of zanamavir or oseltamivir is cost-effective in high-risk populations, but only cost-effective for the general population if influenza prevalence (among ILI patients) exceeds 20% (treatment) or if cross-contamination rates exceed 8% (prophylaxis).
Myth #3 . No other effective PEP therapies exist for influenza-like illness.
As reported in Emergency Physicians Monthly, facemasks and prescribed hand-hygiene can reduce (18% to 4%, Odds Ratio 0.33) the incidence of influenza spread in household contacts if initiated within 36-hours of symptom onset.
Conclusion: Physicians can reduce the spread of influenza by prescribing facemasks and hand-washing to patients and household contacts.

Myth #4 . Health care providers do not require flu vaccinations.
Healthcare worker associated influenza outbreaks can be costly for hospital systems. One systematic review of 18 studies analyzing influenza immunization for healthcare providers demonstrated a reduction in patient mortality at a savings of $20 per vaccine. Yes, immunizing providers reduced patient mortality. Unfortunately, less than 25% of European providers were immunized. In the U.S., only two-thirds of ED staff report high likelihood to receive influenza vaccine each year with physicians (>82%) more likely than nurses (42%) to obtain the vaccination. In Canada only 35% of physicians report compliance with annual influenza vaccinations with 28% believing that adverse affects are common and only 52% believing in the vaccine effectiveness. Additionally, only 27% believed that patients could contract influenza from a healthcare provider, but 58% believed that providers could be infected by patients. Among Pediatric EM specialists, 40% fail to receive annual influenza vaccinations. Similarly, 35% of pre-hospital providers do not receive annual influenza vaccinations with waiting time and costs identified as barriers to compliance. Not surprisingly, vaccinating ED healthcare workers reduces sick-related absenteeism (55% vs. 30%). Multiple pandemic models suggest that vaccination and prompt symptom-based hygiene education is an efficacious and cost-effective strategy to reduce influenza-related mortality.
Conclusion: By vaccinating themselves, healthcare providers can reduce their own morbidity while simultaneously reducing healthcare costs and patient mortality.
Myth #5 . Patients can not be vaccinated in the emergency department
Ever heard of tetanus? Multiple studies over the last 20 years have demonstrated that EDs evaluate multiple high-risk, unvaccinated patients every year. Many lack primary care access. The majority of these patients are willing to be vaccinated. Influenza vaccination in busy EDs is feasible. Of course, there is concern about repeat vaccinations for patients who are uncertain about their status. However, the risk of adverse events are minimal and local information systems can be developed to prevent this unnecessary risk and resource utilization.
Conclusion: Vaccinating high-risk influenza patients (see Table) in the ED is acceptable to patients and imminently feasible.