With recent vancomycin shortages, it’s important to know what other drugs can be used to treat MRSA
With recent vancomycin shortages, it’s important to know what other drugs can be used to treat MRSA
With recent vancomycin shortages, it’s important to know what other drugs can be used to treat MRSA

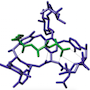
Vancomycin at work: Crystal structure of a short peptide L-Lys-D-Ala-D-Ala (bacterial cell wall precursor, in green) bound to vancomycin (blue) through hydrogen bonds.
Background
The Infectious Disease Society of America has declared this the “Age of MRSA” [1] (Methicillin Resistant Staph Aureus). MRSA has been blamed for tripling ED visits for SSTIs from 1.2 million in 1995 to 3.4 million in 2005 [2]. Vancomycin is one of the medications commonly used to treat MRSA. However, many hospitals nationwide have experienced recent vancomycin shortages. When should vancomycin be used? What other medications could be given instead?

The IDSA’s most recent update (June 2014) [1] of the guidelines for treatment of skin and soft tissue infections (SSTIs) makes a key distinction between the management of purulent infections (carbuncles, furuncles, and abscesses) and of non-purulent infections (necrotizing infections, cellulitis, and erysipelas). The reason for this distinction is that mild/moderate non-purulent infections are typically due to Streptococcal species, while purulent infections, even if there is surrounding erythema, are more often caused by Staphylococcus, and specifically, MRSA. Vancomycin can play an important role in both types of infection in severe cases. However, for mild or moderate infections, vancomycin is not indicated, and even in severe cases, there are several other choices.
Major ED Uses
Severe, purulent or non-purulent SSTIs, MRSA, pneumonia, meningitis, C difficile-associated diarrhea (CDAD), catheter-related infections, infection in seriously ill patients or immunocompromised patients.
In The News
A drug shortage of vancomycin was listed by the American Society of Health-System Pharmacists as of 09/2014 [3], though a shortage is not currently reported by the FDA [4].
How it Works
Vancomycin inhibits polymerization of the cell wall in gram positive bacteria. It has no activity against gram negative bacteria. Staph Aureus can have intermediate resistance to vancomycin (VISA) if it has a thicker cell wall. True resistance (VRSA) comes from the vanA gene, which changes the terminal amino acid in the peptidoglycans that make up the cell wall [5].
Notable History
Vancomycin is a peptide-based, naturally-occurring antibiotic. It was first purified by Eli Lilly and the results published in 1955 [6]. The antibiotic was extracted from a previously unknown bacterium, which was named S. orientalis, collected from a soil sample in Indonesia.
Adverse Events
More than 10% of patients receiving IV vancomycin may experience hypotension, flushing, and an erythematous rash which is referred to as “red man syndrome.” Red Man Syndrome usually can be managed by temporarily stopping the infusion, administering diphenhydramine (Benadryl) 50mg PO or IV and ranitidine (Zantac) 50mg IV, and then restarting the infusion at half the rate, though these recommendations have not been thoroughly studied [7]. Less common complications include nephrotoxicity, ototoxicity, and allergic reactions such as Stevens-Johnson syndrome.
Cautions
Vancomycin is pregnancy class C when given intravenously and class B when given orally. It is secreted in breast milk, but there is little systemic absorption from PO intake by the infant, so it is generally considered safe for breast-feeding mothers [8].
Dose Adjustments
Initial dosing is typically 15mg/kg IV. Patients with renal impairment will require less frequent dosing, but the initial dose can be the same. Keep in mind that the pre-mixed IV bags of 1g would be the correct dose for a patient weighing 67kg (147 lbs). Most adults weigh more than this, so make sure the dose given is weight-appropriate.
Special Features
Vancomycin is not recommended by the IDSA for mild or moderate SSTIs. Neither is there a recommendation to give a single dose of IV vancomycin prior to discharging a patient on oral antibiotics. A single dose does not reach therapeutic levels, but could potentially give rise to side effects, increased ED length of stay, and potential resistance. Antibiotics recommended by the IDSA [1] for mild non-purulent SSTIs include oral penicillin, cephalosporin, dicloxacillin, or clindamycin. For moderate non-purulent SSTIs, i.e. those in which the patient is experiencing systemic symptoms requiring IV antibiotics, penicillin, ceftriaxone, cefazolin, or clindamycin are recommended. For severe non-purulent SSTIs, empiric vancomycin PLUS pipercillin/tazobactam (Zosyn) or vancomycin PLUS imipenem/meropenem are recommended.
In certain instances, patients with non-purulent infections should be switched to the “purulent infections” algorithm, specifically, those with “penetrating trauma, evidence of MRSA infection elsewhere, nasal colonization with MRSA, injection drug use, or SIRS [2].” Patients who are immunocompromised may also require broader coverage.
Regarding purulent infections, for mild cases, incision and drainage alone should be sufficient. For moderate cases, TMP/SMX (Bactrim) or doxycycline can be used empirically. For severe infections, vancomycin is recommended for empiric treatment. However, if your hospital is experiencing a shortage, other agents recommended empirically include daptomycin, linezolid, televancin, or ceftaroline.
Cost
1g of IV vancomycin solution costs around $6-$30, while oral capsules sell for over $30 per 125mg tablet [9].
Christina Shenvi, MD, PhD is an assistant professor in the department of emergency medicine at the University of North Carolina
1. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the infectious diseases society of america. Clin Infect Dis. 2014;59(2):147-159.
2. Infectious Diseases Society of America. Increase in MRSA prompts updated ISDA guidelines for skin and soft tissue infections (SSTI) http://www.idsociety.org/2014_SSTI_Guidelines/#sthash.qHDadnZM.dpuf. Published 06/19/2014. Updated 2014. Accessed 10/1, 2014.
3. American Society of Health-System Pharmacists. Drug shortages. http://www.ashp.org/menu/DrugShortages/CurrentShortages/bulletin.aspx?id=132. Published 09/24/2014. Updated 2014. Accessed 10/1, 2014.
4. U.S. Food and Drug Administration. Current and resolved drug shortages and discontinuations reported to FDA. http://www.accessdata.fda.gov/scripts/drugshortages/default.cfm. Updated 2014. Accessed 10/1, 2014.
5. Arias CA, Murray BE. Antibiotic-resistant bugs in the 21st century–a clinical super-challenge. N Engl J Med. 2009;360(5):439-443.
6. McCormick MH, McGuire JM, Pittenger GE, Pittenger RC, Stark WM. Vancomycin, a new antibiotic. I. chemical and biologic properties. Antibiot Annu. 1955;3:606-611.
7. Weller PF. Vancomycin hypersensitivity. UptoDate. 2014.
8. Hale TW. Medications and mothers’ milk: A manual of lactational pharmacology. 12th ed. Amarillo, TX: Hale Publishing L.P.; 2012
9. Lexicomp Online. Vancomycin: Drug information. UptoDate. 2014.