A Pulmonary Embolism Presenting with a DVT and Sinus Tachycardia
Case Report:
A 60 year-old male with a significant past medical history of chronic obstructive pulmonary disease, morbid obesity and tobacco dependence presented to the emergency department (ED) for treatment of a deep vein thrombosis (DVT) found on an outpatient Doppler ultrasound. He had noticed swelling to his left leg with associated calf tenderness for three days. Doppler ultrasound revealed a left popliteal DVT.
He denied a history of DVT, pulmonary embolism (PE), shortness of breath, chest pain, syncope or dizziness. Aside from his left leg pain and swelling, he denied any symptoms.
Vitals on arrival: blood pressure of 141/70 mmHg, heart rate of 132 beats/min, respiratory rate of 17 breaths/min, afebrile and 96% on room air. On physical exam, he was sitting comfortably, in no acute distress. Cardiopulmonary exam revealed sinus tachycardia without a murmur and with clear lungs bilaterally. His left lower extremity exhibited edema and calf tenderness consistent with his known history of DVT.
Laboratory evaluation revealed a troponin of 0.03 ng/mL (0.00-0.04 ng/mL) and a B Type Natriuretic Peptide (BNP) of 47 pg/mL (0-100 pg/mL). EKG showed sinus tachycardia without any other acute abnormality. Chest radiology was negative for any acute process.
His initial sinus tachycardia could have been attributed to pain, anxiety or his ambulation to the ED. However, given his multiple co-morbidities, including obesity and tobacco dependence, with a known DVT, clinical suspicion arose for a possible pulmonary embolism.
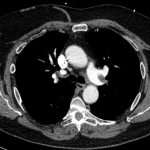
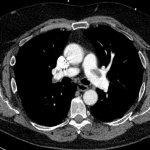
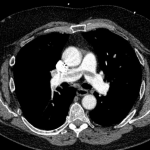
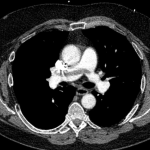
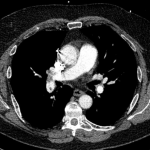
CT angiography of the chest with intravenous contrast was performed.
There is a filling defect straddling the bifurcation of the pulmonary trunk extending into the distal branches of the right lower, right middle, right upper and left upper lobe consistent with a saddle embolism. Bedside echocardiography revealed mild concentric hypertrophy, an ejection fraction of 60%, with normal right ventricular global systolic function, with no evidence for an elevated right ventricular systolic pressure.
The patient was evaluated by cardiology, critical care and interventional radiology regarding treatment. There was no evidence for right heart strain and the patient remained hemodynamically stable with negative cardiac markers. Treatment was decided with heparin over catheter directed thrombolysis or systemic thrombolysis. The patient was admitted to the ICU for his saddle embolism without acute cor pulmonale.
He was transitioned from a heparin drip to Rivaroxaban on day-2. The rest of his hospital course was uncomplicated and he was discharged on day-5. At his one-month cardiology follow-up, he is progressing well, denied any complaints and continues to be compliant with his medications, including anticoagulation.
Discussion:
An acute pulmonary embolism (PE) is relatively common and a leading cause of morbidity and mortality around the world. A saddle embolism involves an embolism straddling the bifurcation of the pulmonary artery. A saddle PE carries with it the potential for catastrophe, as it is a large clot burden that can result in sudden obstructive shock and death.
PE is classified based on the hemodynamic effect, rather than the size. It is important to make the distinction between stable and unstable, as patients that are unstable are more likely to die from obstructive shock.[1] A massive, hemodynamically unstable PE is defined as a systolic blood pressure <90 mmHg for at least 15 minutes, decrease in baseline systolic of 40 mmHg or more for at least 15 minutes, or requiring inotropic support, not due to any other diagnosis besides a PE. A submassive, hemodynamically stable PE is defined as a systolic blood pressure >90 mmHg, mild hypotension that responds to fluid resuscitation, however, with evidence of right ventricular (RV) dysfunction or necrosis of the myocardium.[2]
RV dysfunction has been defined as the presence of at least one of the following:
- RV dilation on CT or echo (RV/LV >1:1), septal bowing, RV hypokinesis with associated normal apical contractility, or an intraventricular thrombus
- Elevated brain natriuretic peptide (BNP) >90 pg/ml.
- New or incomplete right bundle branch block (RBBB), anteroseptal T-wave inversion, anteroseptal ST elevation/depression.[2]
- S1Q3T3 indicating acute cor pulmonale (found in up to 25% of patients diagnosed with a PE).
The frequency of a saddle embolism is about 5% in all patients diagnosed with a pulmonary embolism.[3] Patients may present hemodynamically stable with no evidence of right heart strain, but the proximal thrombus can fragment spontaneously or as a result of treatment. This leads to obstruction of multiple, distal pulmonary arteries, with associated right heart strain and obstructive shock.[3]
In a retrospective study involving 546 patients diagnosed with an acute PE by CT angiography from 2002 to 2003, approximately 14 patients (2.6%) had a saddle PE. The most common presenting symptoms in this group included: dyspnea (72%), syncope (43%), chest pain (21%) and nausea (14%).[4] Important to note is that these were the most common presenting symptoms in a saddle embolism, not for a PE in general, as noted in the PIOPED study.
The most common risk factors identified were obesity (64%), age >60 (29%), malignancy (29%), surgery within three months (29%) and previous embolism (14%). Right heart strain was subsequently diagnosed by echo in only eight of the patients.[4] All 14 of the patients received heparin and warfarin, five received additional therapy, and one received thrombolysis with all of the patients surviving and being discharged from the hospital. [4]
In this study, the clinical presentation varied from the absence of cardiopulmonary symptoms to hemodynamic instability.
A large systematic review was performed involving twenty-eight published studies regarding silent PE in patients diagnosed with a deep vein thrombosis (DVT). A total of 1665 out of 5233 patients (32%) diagnosed with a DVT had a silent PE.[5] The incidence of a silent PE with a coexisting proximal DVT was found to be higher than a coexisting distal DVT.[2] Some studies found that the PE on CT angiography involved up to 60% of the pulmonary artery, with the patient remaining hemodynamically stable.[6]
Overall, a silent PE was identified in 14% of patients <40 years-old, 22% from age 40-70, and 40% >70 years-old.[7] For patients with a saddle embolism or fatal embolism at autopsy, nearly 78% (1902/2448) were undiagnosed or unsuspected.[8] A strength of this systematic review is that it included 1665 patients with a silent PE, however, there was heterogeneity in detecting the silent PE.[5]
In our patient, he was hemodynamically stable, no evidence for right heart strain, leading to the patient being treated with intravenous heparin. Treatment options beyond traditional therapy like heparin includes thrombolysis and localized catheter director therapy.[4] The current consensus among the literature indicates that patients with a confirmed PE or multiple emboli complicated by severe RV dysfunction and hemodynamic instability secondary to an acute massive PE should receive thrombolysis.[9]
Conclusion:
The administration of Alteplase (tPA) in the setting of cardiac arrest is considered a potential life-saving intervention. While many protocols exist for code dosing of tPA, one such protocol comes from University Hospital in San Antonio, TX.
A special note is that this protocol follows for PEA arrest only (most common rhythm in obstructive shock from a PE), with all other rhythms following standard ACLS protocol.[10] With a massive PE high on the differential with a PEA arrest, the physician can consider administering:
- 50 mg tPA IV bolus x 1 and continuing with ACLS
- If ROSC is achieved within 15 minutes, a heparin drip is initiated
- If ROSC is not achieved within 15 minutes, a repeat dose of 50 mg tPA IV bolus x 1 and continuing ACLS for at least 15 minutes
- If ROSC is achieved, a heparin drip is initiated
In a non-arrest state, the U.S Food and Drug Administration has approved 100 mg tPA as a continuous IV infusion over a two-hour period for the treatment of an acute massive PE.[9] Other treatment options for those patients with cardiogenic shock, those who have failed thrombolytic therapy, or have a contraindication, embolectomy can be considered.
The mortality rate in the setting of cardiac arrest approaches up to 95%, with the majority diagnosed at autopsy as the cause of the arrest. Our patient presented a known DVT, sinus tachycardia, and was found to have a large clot burden throughout the pulmonary artery. He had a high potential for acute decompensation and death. With an extremely high mortality in the setting of cardiac arrest, quick recognition, intervention and treatment is key to potentially saving a life.
References:
- Thompson, Taylor B. Overview of Acute Pulmonary Embolism in Adults. December 16, 2016. http://www.uptodate.com/contents/overview-of-acute-pulmonary-embolism-in-adults/print
- Weingart, S. AHA PE Guidelines. July 2011. https://emcrit.org/emcrit/aha-pulmonary-embolism-guidelines-2011/
- Musani, M. “Asymptomatic Saddle Pulmonary Embolism: Case Report and Literature review.” Department in Internal Medicine, St. Joseph Mercy Oakland. 2011 Aug;17(4):337-9. Clinical Applied Thrombosis and Hemostasis Journal. https://www.ncbi.nlm.nih.gov/pubmed/20308228
- Ryu, JH, Pellikka, PA, Froehling DA, Peters, SG, Aughenbaugh, GL. “Saddle pulmonary embolism diagnosed by CT angiography: frequency, clinical features, and outcome.” Respir Med. 2007 Jul;101(7):1537-42. https://www.ncbi.nlm.nih.gov/pubmed/17254761
- Stein, P, Matta, F, Musani M, et al. “Silent Pulmonary Embolism in Patients with Deep Venous Thrombosis: A systematic review.” American Journal of Medicine. 2010 May;123(5):426-31. https://www.ncbi.nlm.nih.gov/pubmed/20399319
- Ryu, J, Pellikka, P, Froehling, D. et al. “Saddle pulmonary embolism diagnosed by CT angiography: Frequency, clinical features, and outcome.” Respiratory Medicine. Volume 101, Issue 7, July 2007. Pages 1537-1542. https://www.ncbi.nlm.nih.gov/pubmed/17254761
- Partsch, H, Kechavarz, B, Mostbeck, A. “Frequency of pulmonary embolism in patients who have iliofemoral deep vein thrombosis and area treated with once or twice daily low molecular weight heparin.” Journal of Vascular Surgery. 1996;24:774-782. https://www.ncbi.nlm.nih.gov/pubmed/8918323
- Hirsch, J, Demilly, P. “Screening of asymptomatic pulmonary embolism by systematic scintigraphy in apparently uncomplicated phlebitis.” Annals of Internal Medicine. 1991; 142:168-170. https://www.ncbi.nlm.nih.gov/pubmed/1854128
- Sekaran, D, Scoccimarro, A, Waseem, M. “Pushing tPA in a code situation: What’s the evidence/when can we make the most difference.” January 5, 2016. http://www.emdocs.net/pushing-tpa-in-a-code-situation-whats-the-evidence-when-can-we-make-the-most-difference/
- Garvin, R, Hughes, D, Fowler, A. “Protocol for tPA Use in Adult Cardiac Arrest.” University Health System. https://www.universityhealthsystem.com/~/media/files/clinical-pathways/protocol-tpa-use-in-adult-cardiac-arrest-2016.pdf?la=en