As you polish off the documentation on your last five patients, the nurse for the closed femur fracture patient asks if you are ready for the pending reduction. Orthopedic surgery is patiently waiting, and the nurse has the Propofol that you requested at the bedside.
The Setup:
As you polish off the documentation on your last five patients, the nurse for the closed femur fracture patient asks if you are ready for the pending reduction. Orthopedic surgery is patiently waiting, and the nurse has the Propofol that you requested at the bedside. Informed consent is obtained. However, as you pause for the Joint Commission recommended pre-procedural “time-out” to mark the correct extremity for reduction and ensure that optimal patient safety conditions have been attained for this particular case, you note a problem. The patient is not on capnography to monitor expiratory carbon dioxide levels. As per your hospital’s new Stop-the-Line policy, you conscientiously halt the procedure before any sedation is administered and ask the nurse why capnography has not been initiated. You are told that the ED’s only functional CO2 detector is now broken (the other two have been missing for months). As you contemplate the options, you search the evidence to support capnography for ED-based procedural sedation.
Search Strategy:
You quickly (less than a minute) conduct a PUBMED search for three terms: “procedural sedation” (523 citations), “emergency medicine” (51,223 citations), and “capnography” (1,195 citations). You combine the three searches, yielding 11 citations. To reproduce this search, go to http://tinyurl.com/2vl594a. An alternative search strategy using two additional MESH headings can be found at http://tinyurl.com/23osuk7.
The Choices:
Postpone the urgent reduction until the capnography unit can be found.
Defer the reduction to the operating room setting where capnography is available.
Proceed with procedural sedation without the benefit of CO2 monitoring.
The Questions:
Does the addition of end-tidal CO2 (ETCO2) monitoring to routine ED procedural sedation care reduce adverse events such as hypoxia, bradycardia, respiratory depression requiring an intervention, hypotension or sedation-related mortality?
The Background:
Providing sedation and analgesia during painful procedures is the standard of care in 21st century emergency medicine. Procedural Sedation/Analgesia (PSA) is the technique of administering sedatives or dissociative agents, with or without analgesics, to induce a state that allows the patient to tolerate unpleasant procedures while maintaining cardiorespiratory function. Potential complications of PSA include hypoventilation, aspiration, and respiratory failure with hypoxic brain injury. Fortunately, EM-based PSA adverse events are rare, and serious events are exceedingly rare. Nonetheless, the Joint Commission has increasingly focused on the safety of PSA in a variety of operative and non-operative settings.
Capnography is the non-invasive measurement of the partial pressure of carbon dioxide in exhaled breath and a capnometer displays the numeric value for ETCO2. Because hypoventilation always precedes hypoxia during PSA, either due to airway obstruction or diminished respiratory drive, ETCO2 monitoring provides an early warning signal permitting time to intervene before the onset of hypoxia. Capnography was developed in the 1940’s and was first described by Dr. Art Sanders in the emergency medicine literature in the 1980’s. Some have hypothesized a role of ETCO2 monitoring to rapidly assess gastroenteritis or chemical-weapons related acidosis, non-invasive positive pressure ventilation success, pulmonary embolism risk stratification or to confirm endotracheal tube positioning during transports. Observational data have suggested that monitoring of ETCO2 during ED PSA detects respiratory depression significantly faster than pulse oximetry alone, but randomized trials have not previously been performed. While the ACEP Clinical Policy for PSA suggests one “consider capnometry to provide additional information regarding early identification of hypoventilation,” Anesthesiology guidelines for non-Anesthesiologists suggest “…monitoring of exhaled carbon dioxide should be considered for all patients receiving deep sedation and for patients whose ventilation cannot be directly observed during moderate sedation.” In anesthesia, ETCO2 monitoring has been standard of care in the operating room for over 25 years. The current study provides the first objective evidence in ED settings that ETCO2 monitoring may improve patient safety during PSA.

Results:
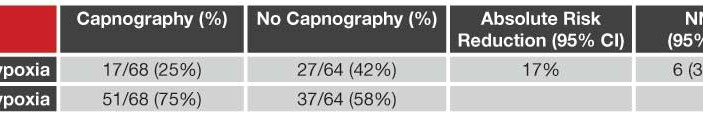
At Albert Einstein Medical Center (Philadelphia) a total of 210 subjects undergoing PSA with Propofol (1 mg/kg then 0.5 mg/kg boluses using ideal body weight) were screened and 132 randomized to intervention (standard monitoring + capnography measuring ETCO2 via nasal cannula using industry-supplied Capnostream 20™) or control (standard monitoring alone) groups. All patients received 3L/minute oxygen and 0.5 μg/kg fentanyl or 0.05 mg/kg of morphine at least 30-minutes prior to the procedure. In the intervention group, research assistants promptly notified the emergency physician when predefined abnormal ETCO2 monitoring results were noted. During the procedure, research assistants noted the time and nature of any interventions for respiratory depression or hypoxia as well as any sedation-related adverse events such as hypotension, bradycardia, arrhythmia, vomiting, prolonged ED stay or avoidable admission. The primary outcome was hypoxia, as defined by an oximetry reading of ≤ 93%. Respiratory depression was defined a priori as ETCO2 ≥ 50 mm Hg, an absolute increase or decrease from baseline ETCO2 ≥ 10%, or loss of the waveform for > 15 seconds.
Overall, 33% (44/132) had at least one hypoxic episode with respiratory depression equally prevalent in both groups (57% intervention group vs. 58% in control group). Capnography-defined respiratory depression was 100% sensitive and 64% specific in predicting hypoxia with the loss of a waveform being the most likely finding to precede hypoxia. Physicians informed of capnography results demonstrated a trend towards performing an intervention to improve respiratory status (35% of capnography group vs. 22% of control group performed a respiratory intervention; 95%
CI -2% to 27%). In patients with hypoxia, the median time from onset of respiratory depression to hypoxia was 60 seconds (range 5 to 240 seconds).
The Bottom Line:
One-third of patients at one urban academic ED undergoing Propofol procedural sedation experience a hypoxic event (oxygen saturation ≤ 93%) and Capnostream 20™ ETCO2 monitoring significantly predicts the development of hypoxia with a Number Needed to Treat (NNT) of 6 and up to 4-minutes advanced notice compared with pulse oximetry or clinical observation alone.
The Caveat:
The investigators do not provide significant demographic descriptors for their patient population such as the proportion with known or suspected obstructive sleep apnea or measurements of co-morbid illness burden, acute illness severity or hospital length-of-stay. Furthermore, in a tight economy with increasing declarations for fiscal constraint, ED leaders recognize that the Capnostream 20™ retails at $4,950 per unit raising the following questions:
What is the value of detecting a respiratory event up to 4-minutes before clinical intuition or oximetry monitoring will currently provide?
What is the Patient Oriented Outcome that Matters (POEM) of a 20-second episode of hypoxia?
How much would a lawsuit cost if a preventable PSA-related brain injury, or death, occurred while NOT using ETCO2 monitoring?
Summary:
Healthy skepticism dictates that the worldly-wise, physician-scientist be neither the first nor the last to adopt a new diagnostic/therapeutic innovation. Pro and con arguments can and have been put forth to support and refute ETCO2 monitoring. This is a single trial that does not demonstrate any patient-oriented outcomes. Yet, EBM encourages physician-scientists to use all appropriate data sources to inform best practices. The weight of observational data and this single controlled trial suggest a theoretical benefit for ED PSA patients, which further trials and cost-benefit analyses will hopefully elucidate. In the meantime, your charge nurse locates a functional capnography unit and you proceed with your reduction uneventfully.
Dr. Carpenter is an assistant professor of emergency medicine, and the director of evidence-based medicine, at Washington University in St. Louis



3 Comments
In the interest of considering the the sum of the evidence I suppose the physician would also have to consider the evidence for and against early reduction of displaced femur fractures. Although I appreciate this article as an assessment of the evidence for end tidal capnography, I think the totality of the case must be considered, not just the evidence for and against one element of that patients care.
Dr. J:
Based on your response, not sure if you are for or against capnography?
I absolutely agree that every clinical encounter represents the sum of hundreds of decision trees that culminate in an outcome that we may or may not anticipate or desire. The only way that I know to make sense of this often confusing network of decisions is to systematically isolate each path and analyze the pros & cons of that route.
In the case of capnography, I have concluded that although the technology is expensive and sometimes unreliable, monitoring exhaled CO2 makes physiological sense (face validity) as a means to improve patient safety. We routinely use it in our ED. However, strictly speaking the evidence doesn’t provide me a firm basis to justify my decision so I’m relying upon my clinical gestalt.
EBM has three components: clinical expertise, patient preferences, and the evidence. Optimally, they should overlap to yield best-practice medicine but in the real world sufficient evidence is often lacking to provide unequivocal answers.
Thanks for your thoughts!
Chris Carpenter, MD, MSc
EPM, Chief Clinical Editor
BEEM, Co-Chair
While I agree that all safety measures that are reasonable should be taken with every patient, we should be careful in how we formulate future studies. Just because capnography picks up these events doesn’t necessarily mean that there is any clinically significant benefit to use. As Albert Einstein once said “not everything that is measured is important,and not everything that is important can be measured”. We must be careful not to give those who regulate us in the name of patient safety half of the scientific data. They likely wont interpret it as well as those of us who actually stop and think about the big picture.