A 45-year-old woman presents to the ED with two days of abdominal pain which was gradual in onset and became severe and diffusely distributed…
She had nausea and vomiting over the past two weeks and stated that her abdominal has become very distended. She reported chills, but denied fever, diarrhea, melena, hematochezia, or urinary symptoms. She reported a 60 lb weight loss over the past three to four months. She states she had a negative HIV test two months ago.
Other pertinent medical history included a past medical history of cirrhosis secondary to alcoholism and hepatitis C, pancreatitis, and anemia. The only surgical procedure that the patient had was a breast lumpectomy in 2003, which demonstrated a benign mass. She had a family history of alcoholism, breast cancer, diabetes, hypertension, and renal disease. The patient has a personal history of drinking two bottles of wine per day (though quit four months prior) and cocaine use. She denied any illicit IV drug abuse. Her current medications included lactulose, spironolactone, thiamine, folate, zantac, and a daily multivitamin.
On physical exam, the patient had a blood pressure of 99/57, heart rate of 99 bpm, respiratory rate of 18 breaths/min, O2 saturation 100%, and a temperature of 36.6 deg C. In general, the patient appeared frail, cachectic, and pale. She had scleral icterus and dry mucous membranes. Her abdomen was distended with prominence of the umbilicus, presence of a fluid wave, dullness to percussion in the lower quadrants, generalized tenderness, and normoactive bowel sounds. There was no caput medusa, Murphy’s sign, McBurney’s point tenderness, or rebound tenderness. The remainder of the physical exam was within normal limits.
A bedside ultrasound was performed to evaluate location of peritoneal fluid and perform an ultrasound assisted aspiration.(see figures 1 and 2)

^Fig. 1 Ultrasound demonstrates large fluid collection in seen in the left lower quadrant of the abdomen

^Fig. 2 Ultrasound demonstrates free peritoneal fluid within the left upper quadrant of the abdomen
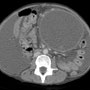
Fine needle aspiration of the left lower quadrant of the abdomen yielded 60mL of black fluid. Within five minutes of the procedure, the patient developed severe abdominal pain. A CT abdomen/pelvis with PO and IV contrast was performed to evaluate for possible perforation. The CT revealed probable pancreatitis with a large pseudocyst (21 cm x 11.4 cm x 12.3 cm) and probable thrombosed pseudoaneurysm versus other cause of localized clot. Some necrosis involving the body and tail of the pancreas was not excluded. Furthermore, cirrhosis, a large fibroid uterus, and ascites with loculation were demonstrated.
The aspirated abdominal fluid yielded the following lab results: lipase 62143, amylase 19623, cell count WBC 3000, albumin 1.1, and RBC 300,000. These lab results were consistent with fluid from pancreatic pseudocyst.
The patient was admitted to the hospital and underwent endoscopic cystogastrostomy drainage of the pseudocyst by the general surgery team.
This patient was ultimately diagnosed with a pancreatic pseudocyst secondary to pancreatitis. Diagnosis of pancreatitis can often be made without imaging in a patient with a predisposition (alcoholism or biliary tract disease), symptoms of nausea, vomiting, and epigastric abdominal pain, along with tachycardia, and amylase and/or lipase three times the upper limits of normal. Of note, serum lipase is only elevated in 70 – 85 % of patients and patients with chronic pancreatitis may not have elevated lipase levels if the pancreas is “burnt out” (1).
Pseudocysts of the pancreas are collections of tissue, fluid, debris, pancreatic enzymes, and blood that typically develop over a period of 4 – 6 weeks after the onset of acute pancreatitis (3). Pseudocysts form in approximately 15 % patients with acute pancreatitis, but also form in patients with chronic pancreatitis and blunt pancreatic trauma. They are pseudocysts because they do not have an epithelial lining. The walls of pseudocysts are made of necrotic tissue, granulation tissue, and fibrous tissue. They are formed by the walls of surrounding structures. Pseudocysts may be located within the pancreas or externally, may be small or large, and may have one or multiple structures (4).
A variety of radiographic modalities may be used to diagnose a pseudocyst. Most commonly, ultrasound and CT scans are use. The benefit of using ultrasound is the ability to perform serial exam to monitor expansion or resolution of the pseudocyst without exposing the patient to repeated doses of radiation. Unfortunately, ultrasound is not as sensitive as CT scan (5). MRI may also be used to aid in characterization of mass, as well as to aid in evaluation of pseudoaneurysm formation.

^Fig. 3 CT scan of abdomen and pelvis demonstrates a large pancreatic pseudocyst left of midline
Although imaging may not be required for the diagnosis of pancreatitis, there are several situations in which imaging should be considered. One patient group who may benefit from imaging are elderly patients in which the diagnosis is in question, given that multiple disease processes may present in a similar manor. Furthermore, patients who have a suspected diagnosis of pancreatitis who develop worsening pain one week after onset of symptoms, peritoneal signs, or hemodynamic instability would also benefit from imaging in order to evaluate for complication of pancreatitis such as pseudocyst, phlegmon, abscess, or necrotizing pancreatitis. In general, imaging may be indicated to confirm or exclude a clinical diagnosis of pancreatitis, evaluate disease severity and for disease complications, or to provide guidance for percutaneous therapy (2).
The majority of pseudocysts spontaneously resolve within 6 weeks after formation, thus conservative management is preferred if the patient’s symptoms are mild and the patient is not actively using alcohol. Furthermore, large sizes of cysts do not mandate drainage. If patients have an increasing size of the pseudocyst or it is complicated by rupture, hemorrhage, or infection, drainage should be performed. If rupture occurs with associated hemorrhage, mortality increases to >60%. Evidence for hemorrhage includes increased size of a mass, a bruit over mass, and an acute decrease in hemoglobin. Pseudoaneurysms develop in up to 10% of patients with acute pancreatitis and are a result of erosion into a blood vessel. The most frequently involved artery is the splenic, followed by inferior and superior pancreatic duodenal arteries (1).
It is important to keep in mind alternative diagnoses of pancreatic fluid collections. If there is concern that the cystic structure is a neoplasm and it is mistakenly treated as a pseudocyst then grave consequences may occur including compromising effectiveness of surgical resection. Findings which make a pancreatic mass less likely to be a pseudocyst include absence of history or signs of acute or chronic pancreatitis or pancreatic trauma, absence of inflammatory changes on CT scan, and the presence of internal septae within the cyst cavity. A high cystic
fluid amylase level favors the diagnosis of inflammatory pseudocyst, but cannot rule out pancreatic cystic neoplasm (4).
If the patient’s condition warrants drainage of pancreatic pseudocyst, less invasive techniques can be used, which include radiologic imaging with percutaneous catheter drainage and endoscopic drainage. Surgical procedures are being used for patients who experienced endoscopic failures, recurrence following successful endoscopic drainage, or do not meet criteria for endoscopic drainage (such as risk of neoplasm or pseudoaneurysm with active extravasation (6).
Resources
- Harrison’s Internal Medicine. 17th Ed. Approach to the Patient with Pancreatic Diseases. Toskes P, Greenberger N. Section 3. Ch. 306. p 2001 – 11. The McGraw-Hill Companies, Inc. 2008.
- Manfredi R, et al. Imaging of acute pancreatitis. Rays. June 2001; 26(2):135-42.
- Bradley EL 3rd. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg 1993; 128:586
- Howell D, Shah R, Lawrence C. Diagnosis and management of pseudocysts of the pancreas. www.UptoDate.com. May 2011.
- Niraj Jani, Murad Bani Hani, Richard. Diagnosis and Management of Cystic Lesions of the Pancreas. Diagnostic and Therapeutic Endoscopy. June 2011. Volume 2011.
- H’ng M W C, Kwek J W, Liau K H, Vu C K F. Cystic pancreatic lesions: a pictorial review and management approach. Singapore Med J 2010; 51(8): 668.



2 Comments
im having a stomach pain for two days now, it comes and go, it seems its getting worse, 1st day that i had the pain, i felt nausea only.
Go to the doctor