This 29-year-old female presented to the emergency department complaining of feeling weak and dizzy. She admitted to being an IV drug user and believed that someone slipped something into her food several days ago while she had been at a truck stop diner. The patient also stated that she was 10 weeks pregnant and recently restarted on methadone for a “past” heroin addiction. The patient complained of diffuse muscle aches and pain in the left hip. She denied fever, chest pain, abdominal pain, nausea or vomiting. She also reported that her urine had been malodorous recently, but denied any other GU or GI complaints.
 Her past medical history was significant for mitral valve prolapse and hepatitis C. She denied using any other medications other than methadone. The patient denied HIV infection, history of tuberculosis or endocarditis. She last injected heroin one day prior to her presentation to the emergency department.
Her past medical history was significant for mitral valve prolapse and hepatitis C. She denied using any other medications other than methadone. The patient denied HIV infection, history of tuberculosis or endocarditis. She last injected heroin one day prior to her presentation to the emergency department.
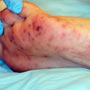
On examination she was somnolent, but arousable. Vital signs were BP 90/60 P120, RR 18 and T 38.9C. HEENT, neck, lung and abdominal exams did not reveal any significant findings other than bilateral conjunctivae injection without discharge and dry mucous membranes (figure 1). Her cardiovascular exam demonstrated a 3/6 systolic ejection murmur at the lower sternal border with radiation to the apex. Her skin had multiple scabs and excoriations without notable superinfection. There were diffuse petechiae on her fingertips, hands, and feet, some of which were painful to palpation (figures 2,3). Her extremities were notable for diffuse muscular tenderness, no joint effusions or decreased range of motion. IV fluids were initiated. A portable CXR was negative for any infiltrates and focused bedside ultrasound to further evaluate her hypotension revealed a 10 week 3 day IUP with a heart rate of 188 bpm with no fluid noted in cul-de-sac or Morrison’s pouch. Subcostal windows demonstrated a hyperdynamic heart without pericardial effusion. Additional laboratory and radiographic findings demonstrated the patient had a pyelonephritis and an elevated total CK which responded to 4 L of normal saline. IV therapy with clindamycin and ceftriaxone were initiated and blood and urine cultures were sent. The patient was admitted for further evaluation and management.

Shortly after admission, the patient’s mental status deteriorated. A head CT was grossly negative and a neurology consult felt this patient had cerebral vasculitis and septic emboli. An ECHO confirmed multiple vegetations attached to the posterior mitral valve leaflet (Figure 4 – click to view). A subsequent CT several days later demonstrated bilateral intraparenchymal hemorrhages. After a stormy course in the intensive care unit, she underwent a replacement of her mitral valve and was eventually discharged to home with a PICC line and IV nafcillin which was continued for 6 weeks. Her blood cultures grew out staph aureus sensitive to all medications tested.
Discussion: This patient had infective endocarditis from IV drug use. Overall, the incidence of infective endocarditis ranges between 2 and 6 per 100,000 population per year with a male to female predominance of 2:1. Despite adequate therapy of acute infective endocarditis, the mortality in the US still remains high at 20 to 30%. IV drug abusers constitute a special risk group and tend to range in ages from 30 to 40 years. In drug users, the tricuspid valve is affected in more than 50% of the cases, followed by the aortic valve in 25% and the mitral valve in 20%. It is thought that the cause of IV drug-related infective endocarditis is related to repeated injection of impure drugs which may cause microtrauma to the tricuspid leaflets. The organisms most often originate from the skin. In IVDA, the mortality is even higher, ranging from 50-60%. Other risk factors for infective endocarditis include preexisting valve abnormality, most often mitral valve prolapse, HIV infection, diabetes mellitus, long term hemodialysis, and poor dental hygiene.
Signs and symptoms of infective endocarditis include fever, chills, rigors (all seen in 25% to 80% of patients), heart murmur (may be absent in right-sided endocarditis), embolic phenomenon with peripheral manifestations in up to 50% of patients, skin manifestations and splenomegaly. Skin manifestations can include petechiae, Osler nodes, splinter hemorrhages, and Janeway lesions. Osler nodes (figures 2) are painful, red nodules found in the pulp of fingers and toes. Janeway lesions (figures 1,2) are macular, blanching, red, nonpainful lesions found on the palms and soles. Petechiae are not specific for IE, but are the most common skin manifestation and can also be seen on the mucous membranes in the mouth or conjunctivae. Peripheral embolic phenomenon includes focal neurological deficits, renal and splenic infarcts, and septic pulmonary infarcts. Other systemic immune reactions such as Roth spots (exudative, edematous hemorrhagic lesions of the retina), glomerulonephritis and arthritis can occur. The rate of embolic events sharply decreases within 1-2 weeks of effective therapy.
The diagnosis of infective endocarditis involves meeting the Duke criteria as established in 1994. The two major criteria include having 2/2 or more positive blood cultures with typical IE microorganisms and/or endocardial involvement as demonstrated by a new valve regurgitation and positive echocardiogram. The organisms involved in endocarditis vary depending on if the infection is acute, subacute or related to IV drugs or prosthetic valves. In acute endocarditis, the major organisms include Staph aureus, strep pneumonia, strep species groups A through G and hemophilus influenza. In IV drug abusers, the most common species include Staph aureus, Pseudomonas aeruginosa, Candida species and Enterococci. Minor criteria for the diagnosis of endocarditis include a predisposing condition or IVDA, fever greater than 100.4 degrees F (or 38 degrees C); vascular phenomenon, immunologic phenomenon, or positive blood cultures not meeting major criteria. Various combinations of the major and minor criteria can be used to make the diagnosis.
Treatment of IE is initially empiric until the cultures are resulted, after which tailored antibiotics are recommended. An initial treatment regimen for IV drug-related endocarditis include nafcillin or oxacillin (2 g IV every 4 hours) plus gentamicin (1 mg/kg every 8 hours) +/- vancomycin (1 g IV every 12 hours) if the patient is at risk for MRSA. For penicillin-allergic patient
s, cefazolin 2 g IV every 8 hours) may be substituted for nafcillin for patients with non-anaphylactic reactions. In all others, vancomycin may be used. The duration of therapy should be 4-6 weeks, but may be as short as 2 weeks in uncomplicated right-sided endocarditis with Staph aureus treated with nafcillin and gentamicin. Two weeks has not been shown to be an effective amount of treatment time for patients on vancomycin only.
Surgical repair of the affected valve may be done if the there is rapid destruction of the valve leaflet with severe aortic or mitral regurgitation, a large vegetation is present (greater than 10 mm), an abscess or fistula develops at the valve ring or in patients with cardiac failure and hypotension or shock.
References:
- Cohen and Powderly: Infectious Diseases, 2nd ed. 2004 chapter 59, endocarditis
- Ferri’s Clinical Advisor 9th ed. 2007 infective endocarditis
- Up-to-date, diagnostic approach to infective endocarditis and medical treatment of native valve endocarditis